Research Experience
Investigating the Effects of Ammonium Fluoride Concentrations on the Surface Microstructure of Anodized Aluminum Films
Abdullah al Rafi, Raihan Ahmed Redoy, Md Shamim Ali, Md. Abu Mowazzem Hossain *, Md Eyasin Hossain
Mar 2025 – Aug 2025
Abstract
Anodizing is an electrochemical process that forms a protective oxide layer on metals, usually aluminum. By applying electricity in an electrolyte solution, a durable coating is created whose thickness can be controlled by process conditions. Anodized aluminum gains improved corrosion and scratch resistance, along with a matte or satin finish for better aesthetics than the untreated aluminum. This study explored the use of platinum as a cathode in the electrochemical anodization of aluminum with various electrolyte solutions. Aluminum served as the anode, and the process was conducted in solutions containing ammonium fluoride (NH₄F), ethylene glycol (C2H6O2), and ammonium sulfate ((NH₄)₂SO₄), with varying concentrations and anodization durations. Scanning electron microscopy (SEM) was used to characterize the oxide layers. The study showed that using platinum as a cathode produced a uniform oxide layer influenced by electrolyte conditions. Higher ammonium fluoride concentrations yielded thicker, more uniform layers with smaller pores, while lower concentrations gave thinner layers with larger, irregular pores. Longer anodization times and lower voltages improved pore uniformity. Among the samples, Sample-3 (0.5 wt.% NH₄F for 2 hours) provided the most optimal microstructure, while Sample-2 outperformed Sample-1 in pore consistency. These findings contribute to the development of materials and coatings for applications such as biomedical implants and electronic devices.
Objective
The objective of aluminum anodization is to investigate how NH₄F concentrations affect the morphology and properties of anodized aluminum, including the structure and composition of the oxide layer. This study also aims to assess the interactions of ethylene glycol, ammonium fluoride, and ammonium sulfate during the anodization process and determine the optimal ammonium fluoride concentrations for desired anodization rates. The goal is to create a durable, corrosion-resistant aluminum oxide coating by passing an electric current through an electrolyte, with parameters like voltage, current density, and electrolyte composition influencing the thickness, hardness, color, and texture.
Graphical Representation
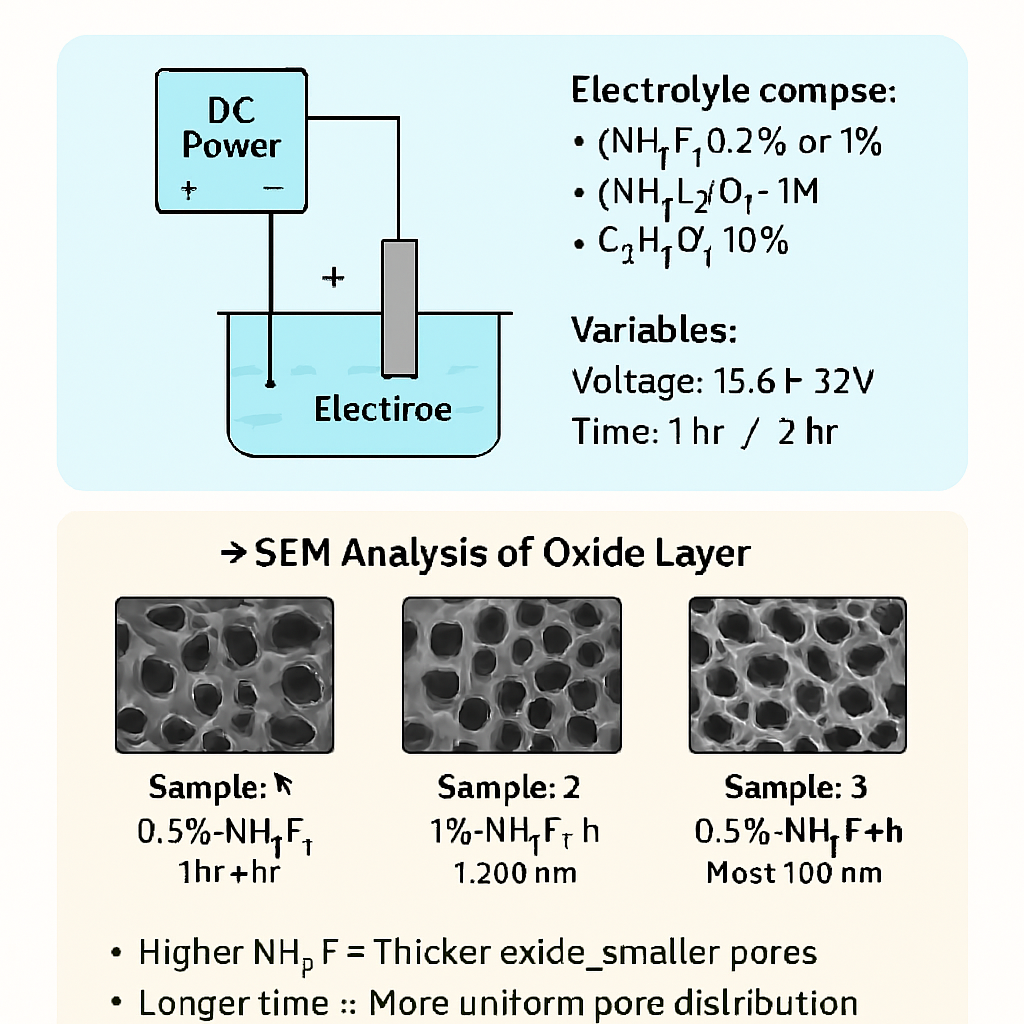
Key Points
- Developed three different samples of anodized aluminum by varying the concentration of Ammonium Fluoride (0.5 wt%,1 wt%), voltage applied (high, low, moderate), and anodization time (1 hour, 2 hours).
- Compared the microstructure of different samples by the results of Scanning Electron Microscopy (SEM) test images.
- Used ImageJ software to find out the pore size distribution of the three samples.
